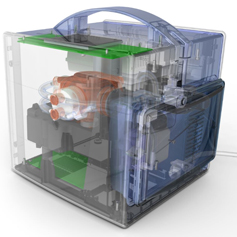
MEDICAL DEVICES &
LAB INSTRUMENTS
From concept development through the most complex technical implementation, PDT's medical device development experience encompasses a broad range of end user communities and applications. Our portfolio spans connected devices, patient monitoring, therapeutic devices, surgical instrumentation, lab instruments, point-of-care testing (POCT), diagnostics equipment, infection prevention, drug delivery systems, assistive technologies, and disposables.
Research
Contextual exploratory and in-depth research fuels our design work with a firm understanding of end users and their in-field requirements. We gain access to global institutions with high barriers to entry - hospitals, physician offices and laboratories - to observe products in their real world settings.
Human Factors
We leverage insights from user observation to create ergonomic solutions, optimizing comfort and minimizing repetitive strain injuries.
Industrial Design
PDT's strategic research becomes the foundation for inspired, aesthetically balanced solutions for today's demanding medical market.
System and Process Design
PDT understands how to integrate the natural flow of tasks, activities and outcomes into intuitive design solutions at every level of interaction and experience.
User Experience Design
We design on-screen interfaces that encourage engaging, intuitive and safe operation. The right user experience vastly improves procedural outcomes, increases efficiency and minimizes training expenses.
Mechanical Engineering
Our design and engineering teams solve complex challenges in thermodynamics, ingress protection, fluidics, pumping systems and more, always with an eye toward cost-effective production.
Electrical Engineering & Connectivity
Life science devices are getting smarter and increasingly need to communicate within a larger ecosystem. PDT's heritage in telecom and consumer electronics makes us a natural development partner for today's medical technology.
Software Development
Our software and user experience teams work in concert to implement graphical interfaces and applications for multiple platforms, including iOS, Android, and Windows-based systems.
Prototyping
PDT's in-house prototype shop is staffed by highly technical craftspeople who fabricate precision models for testing, evaluation, and marketing purposes.
OUR PROCESS
Our team appreciates the need to integrate front-end creative insight into the procedural rigors of medical device development and contract manufacturing. With quality, control and documentation procedures fully integrated into all of our disciplines, PDT was among the first ISO 13485 certified product development firms in the world with quality, control, and documentation procedures fully integrated into all disciplines.
Our teams follow 60601 guidelines for efficient regulatory agency and Ministries of Health approval, and through extensive design documentation can support all FDA submission opportunities. Integrated risk assessment / FMEA (Failure Mode and Effects Analysis), FTA (Fault Tree Analysis) and verification / validation testing then guides your program through production, including complete support in design transfer to our manufacturing facility or select FDA-approved manufacturers.
Manufacturing & Assembly
Our large staff of full-time, trained, credentialed professionals serve as a partner in designing, optimizing, assembling, and shipping complex medical devices. Our global sourcing team also leverages a trusted network of partners in Asia.
Quality Assurance
Our on-site lab conducts drop, ball drop, ingress, life cycle and immersion testing, high speed photography and more.
RELATED ARTICLES
The Changing Point of Care Testing Landscape >
Pharmacies as the Point of Care Nexus >
Connected Health Product Development >
RELATED PROJECTS
Connected Blood Glucose Meter
Negative Pressure Wound Therapy (NPWT) Device
Next-Generation Peristaltic Pump
Connected Autoinjector System
Electrosurgical RF Generator
Connected Drug Delivery Device
Next-Generation Ambulatory Infusion Pump
HOW CAN WE HELP YOU?
EMAIL US
FOLLOW US